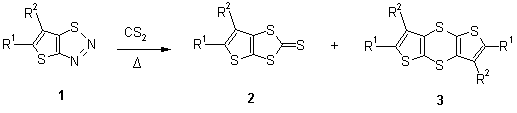
Synthesis of Thieno [2,3-d]-1,3-dithiol-2-thiones from Thieno [2,3-d]-1,2,3-thiadiazoles: Matryoshka-type autoclave for high-temperature, high-pressure thermolytic microscale reactions.
| Overview | The Matryoshka Autoclave |
|
 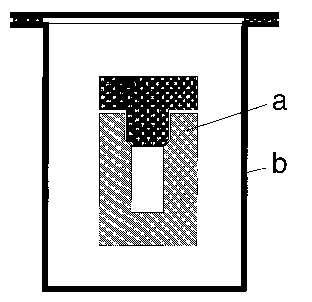
a: inner TEFLON autoclave
|
Condensed 1,3-dithiole-2-thiones are valuable intermediates in the preparation of tetrathiafulvalene (TTF) - type organic metals [2]. Some of these compounds also exhibit antifungal activities [3]. Known methods of preparing thieno[2,3-d]-1,3-dithiol-2-thiones include either the annelation of the thiophene ring onto suitably substituted 1,3-dithiol-2-thiones [4,5] or the cyclization of thiophene thiole derivatives [6,7,8]. thieno[2,3-d]-1,3-dithiol-2-thione has been synthesized by treatment of O-ethyl-S-(2-oxotetrahydrofuran-3-yl)dithiocarbonate with phosphorous sulfide or sulfur dehydrogenation of 4,5-dihydro-thieno [2,3-d]-1,3-dithiole-2-thione [9]. The isomeric thieno[3,4-d]-1,3-dithiole-2-thione is formed as a side product by thermolysis of 4,6-dihydrothieno[3,4-d]-1,2,3-thiadiazole in the presence of an excess of carbon disulfide (6h, 170°C) [10].
In continuation of our previous work on the synthesis of 1,3-benzodithiole-2-thiones [11] we have investigated the reaction of thieno[2,3-d]-1,2,3-thiadiazoles [12] with carbon disulfide. The carbon disulfide thermolysis reactions were performed using a micro-scale, Teflon-autoclave within a 2L steel autoclave (Fig. 1). By filling both the inner and outer autoclaves with the carbon disulfide solvent, one could expect, that on heating only a relatively small pressure difference would exist between the inside and outside of the Teflon autoclave, while the pressure within the steel autoclave was between 23 and 35 bar. Indeed, by using this procedure, reactions on a 140-840 mg scale could be performed routinely in a Matryoshka-autoclave, with the added advantage, that contamination of the product by impurities (due to reaction of the autoclave with carbon disulfide) is avoided.
Fig. 1a: Matryoshka Dolls and Matryoshka-Type Autoclave
|
|
|
a: inner TEFLON autoclave
b: outer steel autoclave
Dimensions: diameter: 50 mm,
overall height: 90 mm, |
2 L steel autoclave |
The reaction temperature plays a critical role. Although the optimal temperature for the conversion of benzo-1,2,3-thiadiazole to 1,3-benzodithiole-2-thione was found to be 220±5°C [13] with 37% yield of 2a formed; below 220°C, 1a was mostly unreacted. Increasing the temperature to 230-240°C increased the yield of 2a to 67%. Under similar reaction conditions the cyanide derivative (1f) gave, in addition to the thieno[2,3-d]-1,3-dithiole-2-thione (2f), the 1,4,5,8-tetrathia-s-indacene (3f). The formation of the products 3d and 3f can be explained via head-to-tail dimerization of the 1,3-dipolar intermediate [14] (Scheme 1).
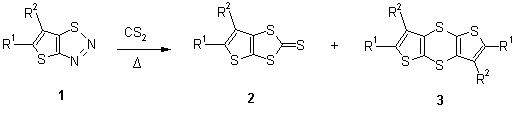
5-Phenylthieno[2,3-d]-1,3-dithiole was not detected on reduction of 2d using the boron hydride/dimethyl sulfide complex. Instead, the ring-cleaved dithiole (4a) was formed and characterized as the dimethylated compound 4b (Scheme 2).
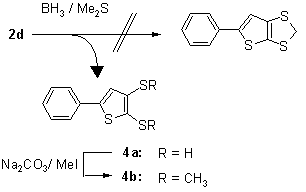
This behavior is in contrast to the analogous conversion of 1,3-benzodithiole-2-thione to benzo-1,3-dithiole, which was produced routinely with yields of 88-92% in our laboratory [15].
On the other hand, 5-ethoxycarbonyl-6-methylthieno[2,3-d]dithiole-2-thione (2g) behaved analogously to benzo-1,3-dithiole-2-thione on treatment with dimethyl sulfate followed by tetrafluoro-boric acid. The crystalline salt 6 was then reduced to 7a using sodium borohydride in CH3CN/THF. When ethanol was used for the reduction as described for similar reactions [16] a mixture of 7a and 7b was formed:
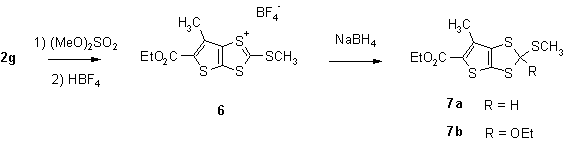
2-Methylthio-thieno[2,3-d]-1,3-dithiolium salts (e.g. 6)
could serve as starting materials for a host of synthetically useful reactions [17].
We have presented a facile route for the formation of thieno[2,3-d]-1,3-dithiol-2-thiones and developed a nested-type autoclave that allows to use Teflon autoclaves even under high pressure.
1. Matryoshkas (=Matriushka, Matrushka) nesting dolls were introduced at the end of the 19th century by the Russian artist Sergei Maliutin who painted a wooden doll as a peasant girl with a rooster. Inside of this hollow doll there were seven more dolls all painted differently (Fig. 1).
2. Bryce, M.R. Tetrathiafulfalene (TTF) and their selenium and tellurium analogs (TSF and TTeF): electron donors for organic metals. Aldrichimica Acta 1985, 18, 73-78.
3. See e.g. Chinothionat: Büchel, K.H., Ed.; Pflanzenschutz und Schädlingsbekämpfungsmittel Georg Thieme Verlag, Stuttgart 1977.
4. Dölling, W.; Vogt, A.; Augustin, M. Synthesis of 1,3-dithiole-2-one derivatives, 2-ethylthio-1,3-dithiolium tetrafluoroborates and thieno[2,3-d]-1,3-dithiole-2-thiones. Z. Naturforsch. 1991, 46b, 133-138.
5. Dölling, W.; Augustin, M.; Ihrke, R. A simple method for the preparation of 6-amino-thieno[2,3-d]-1,3-dithiol-2-thiones. Synthesis 1987, 655-657.
6. Litvinov, V.P.; Dzhumaev, I.A.; Condensed heteroaromatic 1,3-dithiole-2-thiones and their selenium analogs. Izv. Akad. Nauk SSSR, Ser. Khim. 1982, 717-718.
7. Litvinov, V.P.; Dzhumaev, I.A.; Zolotarev, B.M. Synthesis and mass-spectrometric study of condensed thiones-precursors of thiophene-type heterofulvalenes. Izv. Akad. Nauk SSSR, Ser. Khim. 1983, 2105-10.
8. Rasheed, K.; Warhentin, J.P. Thermal decomposition of dinitropyridyl and dinitrothienyl dithiocarbamates and t-butyl trithiocarbonates. J. Heterocycl. Chem. 1981, 18, 1581-1585.
9. Engler, E.M.; Patel, V.V.; Andersen, J.R.; Schumaker, R.R.; Fukushima, A.A. Organic Metals. Systematic molecular modifications of hexamethylene tetraheterofulvalene donors. J. Am. Chem. Soc. 1978, 100, 3769-3776.
10. a) Rovira, C.; Santalo, N.; Veciana, J.; Bis(thiodimethylene)-tetrathiafulvalene (BTDM-TTF). A new π-electron donor with relevant oxidation properties. Tetrahedron Lett. 1989, 30, 7249-7252. b) Rovira, C.; Veciana, J.; Santalo, N.; Tarres, J.; Cirujeda, J.; Molins, E.; Llorca, J.; Espinosa, E. Synthesis of Several Isomeric Tetrathiafulvalene p-Electron Donors with Peripheral Sulfur Atoms. A Study of Their Radical Cations. J. Org. Chem. 1994, 59, 3307-3313.
11. Jordis, U.; Rudolf, M. Conversion of cyclic trithiocarbonates to thioacetals, including 1,3-dithiane, by reduction with diisobutylaluminium hydride (DIBAL). Phosph. Sulf. 1984, 19, 279-283.
12. Leading references for the preparation of thieno [2,3-d]-1,2,3-thiadiazoles: a) Stanetty, P.; Gorner, E.; Mihovilovic, M.D. An improved synthetic approach to thieno-[2,3-d]-1,2,3-thiadiazolecarboxylates via diazotization of aminothiophene derivatives. J. Heterocycl. Chem. 1999, 36, 761-765. b) Stanetty, P.; Mihovilovic, M.D. A synthetic approach to methyl thieno[2,3-d][1,2,3]thiadiazolecarboxylates via diazotization. Monatsh. Chem. 1999, 130, 573-580. c) Stanetty, P.; Gorner, E.; Mihovilovic, M.D. An improved synthetic approach to thieno[2,3-d]-1,2,3-thiadiazolecarboxylates via diazotization of aminothiophene derivatives. J. Heterocycl. Chem. 1999, 36, 761-765.
13. U. Jordis, unpublished results.
14. Huisgen, R.; Weberndörfer, V. 1,3-Dipolare Additionen der Thioketocarbene. Experientia 1961, 17, 566.
15. Jordis, U. Hydride reduction of 1,3-benzodithiole-2-thiones and -selones. J. Chem. Res. (S) 1986, 432; J. Chem. Res. (M) 1986, 3401.
16. Bryce, M. R.; Coffin, M. A. J. Org. Chem. 1992, 57, 1696-1699.
17. Brown, C. A.; Miller; R. D.; Lindsay, C. M.; Smith, K. Generation of 2-lithio-(2-methylthio)-1,3-benzodithioles, new carbonyl carbanion equivalents, and their application to the synthesis of unsymmetrical hexaorthooxalates. Tetrahedron Lett. 1984, 25, 991-994.
Nakayama, J. The chemistry of 2-alkoxy-1,3-benzodithioles and 1,3-benzodithiolium salts. Reactions and synthetic applications. Sulfur Rep. 1985, 4, 159-194.
Aldoshina, M.Z.; Atovmyan, L.O.; Goldenberg, L.M.; Krasochka, O.N.; Lubovskaya, R.N.; Lubovskii, R.B.; Merzhanov, V.A.; Khidekel, M.L. A new series of radical-cation salts based on asymmetrical ethylendithiodimethyltetrathiafulvalene (EDTDM-TTF). J. Chem. Soc., Chem. Commun. 1985, 1658-1661.
18. Simchen, G. Eine neue allgemeine Heterocyclensynthese durch Alkylierung von Nitril-Halogenwasserstoffaddukten. Habilitationsschrift Universität Stuttgart. 1968.
19. Hennecke, H. DBP 1023464 (1958); C.A. 54: 5704e (1960).
20.
Gewald, K.; Hain, U.; Madlenscha, M. 2,3-Heterocondensed thiophenes from
substituted 2-aminothiophen-3-thiole. J. prakt. Chem. 1988, ,
866-872.